Testing Concentration and Coating Uniformity of Antimicrobial-impregnated Catheters
By Adison Fryman, Application Scientist, Ocean Optics
When a family member recently required catheterization following cancer surgery, he faced an unexpected and potentially lethal consequence: an infection that quickly turned septic, placing him in more immediate danger than his cancer diagnosis. Although our loved one has since recovered from sepsis, his infection persists nearly three months later.
Unfortunately, this story is far too common. Many medically implanted devices are part of standard health care, and each comes with risk of an infection. In 2009, the Centers for Disease Control and Prevention (CDC) issued best-practices guidelines for catheter sanitation, placement and timely removal, as well as the use of antimicrobial/antifungal impregnated catheters. While some areas have since improved, the overall results have been mixed, suggesting the need for continued research and diligence (Figure 1).
Figure 1. Implant infections acquired during treatment typically increase hospital stays by several days – at the cost of about $10,000 per day (1) — and may result in several weeks of IV antibiotics. A simple overnight procedure can turn into a months-long battle with infection, inflicting on the patient more pain, suffering and expenses than originally anticipated.
In this article, our focus is on catheters. I will describe how one manufacturer of antimicrobial-impregnated catheters has traded high performance liquid chromatography (HPLC) testing for onsite modular reflectance spectroscopy to improve and streamline its quality control processes.
Background
Implanted medical devices are everywhere, with central lines or catheters used in chemotherapy, blood transfusions, intravenous (IV) fluid administration, dialysis and IV antibiotics. Preventative measures are the most effective way to avoid device-related infection, but are not enough. As an additional measure, some catheters contain antimicrobial or antifungal additives to help prevent biofilm formation and subsequent infection. That’s important because some experts report nearly 1 in 20 people contracts an infection during medical care. These infections can be deadly: for those that become bloodstream infections, 25% of affected patients will die. (2)
Application Overview
A leading medical device manufacturer recently utilized our Lab Services capabilities to assess the use of optical sensors as part of their product development and quality control for antimicrobial-impregnated catheters.
During the customer’s antimicrobial-impregnated catheter manufacturing process, the catheter tubes are coated with a polymer solution containing a suspended antimicrobial agent that has a chromophore with a specific signature. This coating is cured, solidifying the antimicrobial agent on the exterior of the catheter.
Visual inspection can identify some coating variation along the length of the product. But to ensure that minimum antimicrobial concentrations are present and uniform coatings have been applied, a liquefied sample of the cured antimicrobial layer is sent to an outside lab for HPLC quantification. This process is time consuming, expensive, destructive to the product and does not guarantee a uniform layer (Figure 2). The customer required an onsite system that could determine the concentration and coating uniformity quickly, accurately and with no sample destruction.
Figure 2. For hospitals, preventing catheter-associated infections has financial consequences. That’s because the Centers for Medicare & Medicaid Services will not fully reimburse hospitals for Medicare/Medicaid patients that have central line and catheter-associated bloodstream infections. (4)
Experimental Setup
To measure antimicrobial concentrations at high spectral resolution, we configured a setup comprising an Ocean HDX-VIS-NIR (350-925 nm) spectrometer, an HL-2000-HP high-power tungsten halogen lamp and a Q400-7-UV-VIS reflection probe mounted in a ring stand for repeatability. The Ocean HDX has remarkably low stray light with great thermal stability to give the best possible signal to noise ratio performance, making this high definition spectrometer perfect for developing a concentration curve and determining sample concentrations.
To determine if the Ocean HDX spectrometer setup could be substituted for HPLC yet achieve comparable results, we measured reflectance spectra from several locations of 4 samples with increasing concentrations and one clear (blank) control sample. As you can see in Figure 3, the measurements across the locations were repeatable for each concentration and a clear difference between each concentration is present.
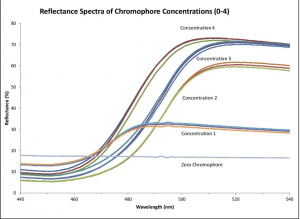
Figure 3. We used an Ocean HDX-VIS-NIR (350-925 nm) spectrometer to measure the reflection of coatings used on antimicrobial-impregnated catheters. Repeatable coating concentration and uniformity results ensure catheter integrity.
The average of each concentration spectra was taken and then divided by the clear reference sample spectrum. This resulted in the average relative reflectance of each concentration. These values plotted against the HPLC concentration data revealed an R2 correlation value of .9924 (Figure 4). This striking correlation between relative reflectance of an Ocean Optics optical sensor and the HPLC-determined concentrations empowered the customer to implement this setup into their quality control process.
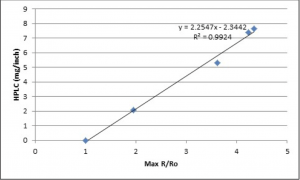
Figure 4. As this graph demonstrates, the Ocean Optics fiber optic spectrometer performed as reliably as the testing lab HPLC system when measuring coating concentrations.
Modular reflectance spectroscopy has proven to be a rapid, cost-effective alternative to HPLC and chemical analysis in many other applications. Learn more about reflectance sampling via the “More info” graphic below.
More info about rapid alternative to HPLC
Discussion
The medical implant business is large and getting larger, with annual sales in 2018 ($400 billion) more than twice what they were in 2000 ($118 billion) (3). As we move into the future of medically implanted devices as part of our everyday lives, ordinary health monitoring devices such as continuous glucose monitors, pacemakers and insulin pumps will become the norm for ongoing treatment of chronic diseases. Preventing infection will become increasingly important to healthcare providers, insurers and patients alike.
To ensure that the increased risk of infection does not outweigh the benefits of these life-saving devices, manufacturers are going to have to deliver the highest quality, safest and cleanest products they can. Today, using robust optical sensing tools and deep application knowledge, Ocean Optics addressed one customer’s need for a reliable onsite concentration and coating uniformity solution. This solution could tomorrow evolve into an inline optical sensor that could detect concentration levels at real-time production speeds.
References: Work Cited
- Health Coverage Protects You from High Medical Costs.” HealthCare.gov, A Federal Government Website Managed and Paid for by the U.S. Centers for Medicare & Medicaid Services, www.healthcare.gov/why-coverage-is-important/protection-from-high-medical-costs/.
- “CDC Vital Signs.” 1 Mar. 2011. Making Health Care Safer, Reducing bloodstream infections. https://www.cdc.gov/vitalsigns/pdf/2011-03-vitalsigns.pdf
- Hallman, Ben, et al. “Medical Devices Harm Patients Worldwide As Governments Fail On Safety.” ICIJ, ICIJ, 7 Jan. 2019, www.icij.org/investigations/implant-files/medical-devices-harm-patients-worldwide-as-governments-fail-on-safety/.
- “Hospital Acquired Conditions.” CMS.gov Centers for Medicare & Medicaid Services, 30 July 2018, www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HAC/Hospital-Acquired-Conditions.html.
Additional Sources
- Percival, S. L., et al. “Healthcare-Associated Infections, Medical Devices and Biofilms: Risk, Tolerance and Control.” Journal of Medical Microbiology, vol. 64, no. Pt_4, 2015, pp. 323–334., doi:10.1099/jmm.0.000032. http://www.microbiologyresearch.org/docserver/fulltext/jmm/64/4/323_jmm000032.pdf?expires=1543864054&id=id&accname=guest&checksum=743F23E2A331771F301419B45ADB749B
- Joel Rosenblatt, Issam Raad. “WO2014172569A2 – Antimicrobial Catheters.” Google Patents, Google, 18 Apr. 2013, patents.google.com/patent/WO2014172569A2.
- O’Grady, et al. “Guidelines for the Prevention of Intravascular Catheter-Related Infections.” OUP Academic, Oxford University Press, 1 May 2011, academic.oup.com/cid/article/52/9/e162/319981.
- St., 24/7 Wall. “The 11 Most Implanted Medical Devices In America.” Business Insider Australia, Business Insider Australia, 19 July 2011, www.businessinsider.com.au/the-11-most-implanted-medical-devices-in-america-2011-7.
- “Why Would I Need A Central Line?” GenesisHealth, www.genesishealth.com/care-treatment/cancer/advanced-treatment/chemo/ports/.
- Whitlock, Jennifer, et al. “Reasons to Place a Central Line in a Patient.” Verywell Health, Verywellhealth, www.verywellhealth.com/central-lines-why-is-a-central-line-necessary-3156818.